Two weeks ago, I wrote about the Elephant Toothpaste experiment I did with my students. A few readers who read that post were curious about how to conduct the experiment and what happens in it. Hence, this follow-up post on the Chemistry behind the experiment. Incidentally, my lesson plan for the next Chemistry class followed the same thought process.
Chemical reaction
The chemical reaction that produces the ‘Elephant Toothpaste‘ effect is – decomposition of Hydrogen Peroxide. Hydrogen Peroxide, in the presence of a catalyst, decomposes rapidly into Water and Oxygen. The chemical equation is as shown in the figure below.
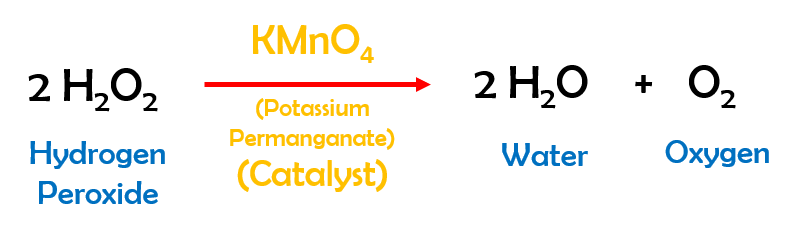
The catalyst we used in our experimental setup was Potassium Permanganate (KMnO4). Other suitable catalysts are Yeast and Potassium Iodide (KI) among many others.
We used Hydrogen Peroxide (6% v/v solution in water) and crystalline Potassium Permanganate; both are available in Medical stores.
The hydrogen Peroxide solution is generally unstable and undergoes slow decomposition in the presence of sunlight. That is why concentrated solutions are stored in dark coloured containers. In the presence of a catalyst, however, the decomposition reaction speeds up. This acceleration is responsible for the ‘Elephant Toothpaste’ effect.
Safety precaution: Hydrogen Peroxide in its pure form is highly unstable and should be handled only by experienced professionals in a laboratory setting.
Chemistry to show-biz
On its own, the chemical decomposition of aqueous Hydrogen Peroxide is quite unspectacular. It happens gradually and because the products are Water and Oxygen, there is no noticeable change either.
To convert this into a visual treat, additional materials are used. In this experiment, the catalyst, food colouring, liquid soap, and the shape of the reaction container play different roles in creating the visual effects.
The visual effects can be varied depending on the choice of catalyst and concentration of Hydrogen Peroxide solution. Watch these videos below to observe different forms of this effect.
Do It Yourself
Here is a simple step by step guide to conduct this experiment at home.
Ingredients
- Hydrogen Peroxide (6% v/v solution)
- Potassium Permanganate crystals
- Food colouring
- Liquid soap (dish-washing liquid is best)
- Empty container (a ½ litre transparent plastic bottle or a tall transparent glass of ½ litre capacity)
Safety precautions
- Conduct it with adult supervision only
- Avoid direct contact of Hydrogen Peroxide and Potassium Permanganate with skin
- Have children observe from a safe distance (preferably 3 feet)
Procedure
- Place the container on a flat surface (table, ground, or a flat tray) – Outdoor location works best; if indoors, choose a well-ventilated area
- Add ¼ teaspoon of food colouring (10-12 drops if in liquid form) into the container
- Add 1 teaspoon of liquid soap
- Pour ~150 ml of Hydrogen Peroxide solution
- Shake well to mix the colour
- Add ½ teaspoon of Potassium Permanganate
- Shake the container gently
- Step away and observe
Note: Because Potassium Permanganate has a vivid purple colour, it generally masks the colour of food colouring. You could try the same experiment with 2 teaspoons of yeast to remove this colour masking effect.
Clean Up
- After the reaction is complete, clean up the container with water and soap
- Clean up the reaction area with water and soap as well (table, tray, floor etc.)
- Do not use the container again for any food or drink
We hope you enjoyed learning the Chemistry behind the experiment. We would love to hear about your experience conducting this at home with your kids. Do share your feedback, photos, and videos with us.
Until next time, continue to stay curious!
*************************************************************************************************************
You can find other articles related to Science, Maths, and Education on our blog.
*************************************************************************************************************

Have you availed the exciting discount offer on the Phonics certification programme yet? Reach out to us at +91 63612 02395 to know more.
Also check out our new learning kits in the NumberNagar® store. The learning kits are a great gifting option for children. Gift the joy of learning to someone in your life.
Featured Image credits: deepakrit from Pixabay
Dr. Soumya Sreehari
Latest posts by Dr. Soumya Sreehari (see all)
- To drink water or not to drink – that is the question - 11 June 2021
- Puzzles for fun and learning - 28 May 2021
- A questioning mind is a thinking mind - 14 May 2021
- Play and learn having fun with words - 7 May 2021
- 4 lessons to learn from the Montessori method - 30 April 2021

