When we think about applications of Science in everyday life, Chemistry in cooking emerges as a natural extension. Cooking is one of the exhaustive applications of Chemistry in our everyday lives. When I was in college, Physical Chemistry was a hard nut to crack for me, especially Thermodynamics. I struggled with the concepts and did the bare minimum work required to pass the exams. It was only in the advanced stages of my academic journey that I developed some appreciation towards it. When I became a teacher, I put in extra effort to make this concept simpler for my students to understand.
A few days ago, while I was cooking dinner, I was spontaneously thinking of Thermodynamics. That thought inspired me to write this article. Chemistry in cooking is indeed intriguing. Here I describe 2 concepts of Thermodynamics that are observed in cooking.
Phase diagram
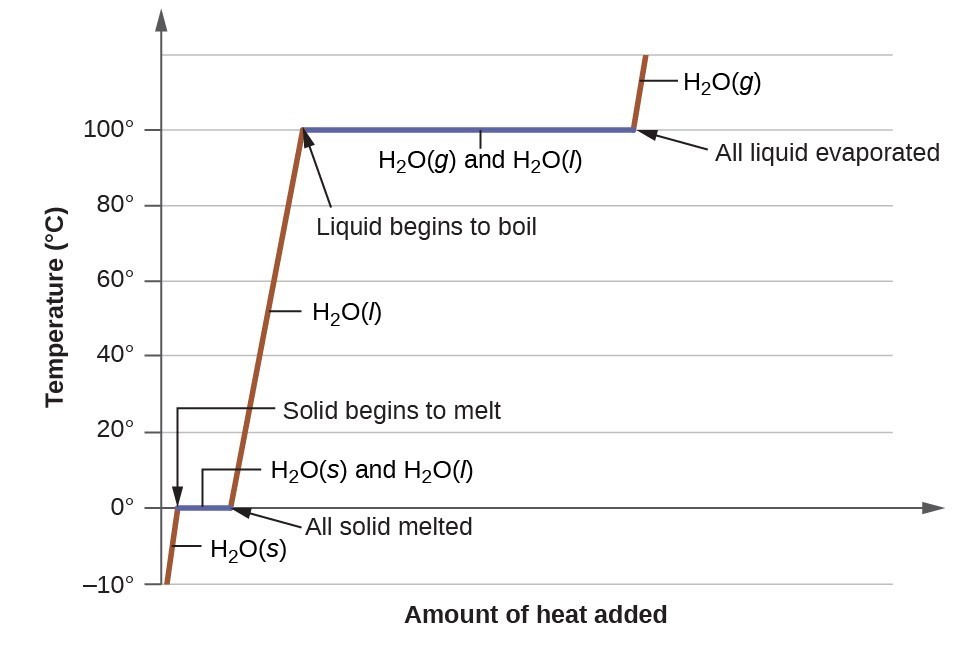
A simple form of a phase diagram is shown in the graph below. This appears in high school Chemistry. The graph describes the change in the state of water as a function of heat provided and increase of temperature.
The graph consists of two parts for every change in the state: a flat line and a slanting line. The flat line part is at specific temperatures. These are the temperatures at which change of state occurs – melting point and boiling point.
As we provide heat to water, the temperature of water increases. When it reaches 0 degrees Celsius (melting point), the temperature remains constant until all ice is converted to liquid water. Only then, the temperature of liquid water increases again. Once it reaches 100 degrees Celsius (boiling point), the temperature remains constant until all the liquid water has evaporated to steam. Then the temperature of steam increases again.
The phase diagram is strictly applicable to pure substances. However, the concept of heat and temperature increase can be extended to cooking. Let us look at an example.
When you cook rice in an open vessel, initially the heat provided is used to raise the temperature of water to boiling point. At that point, the temperature remains constant and the heat is used to cook the rice. Until all the rice grains are cooked, the temperature does not increase again, nor does all water evaporate. Once all the rice grains are cooked, if you continue to heat the vessel, the residual water evaporates, and the bottom of the vessel gets charred.
Have you observed this? If not, pay attention next time. This is also the reason why it is enough to check one grain of rice to know whether the rice is cooked or not.
Gay Lussac’s Law
This law is one of the many gas laws that governs the behaviour of gases within the parameters of Pressure, Temperature, and Volume. The law states that:
“For a gas of fixed mass and fixed volume, the pressure of the gas is directly proportional to its absolute temperature.”
P α T (Pressure is proportional to Temperature)
This law is strictly applicable to gases. However, it can be extended to cooking. Can you guess where? It is to the ubiquitous presence in many kitchens – the pressure cooker. When you cook rice in a pressure cooker, the volume of the container is constant. As heat is provided, the temperature increases, and therefore the pressure inside the container increases. The rice gets cooked at high pressure.
Cooking is a beautiful integration of Science and Art
The concepts I explained above are only a minute sample of the myriad concepts of Science that make cooking possible. They are not straightforward and isolated. Several simple and complex scientific concepts work in combination at every stage of cooking.
While the chef contributes the art to cooking, Mother Nature contributes the science. It is a beautiful integration to be savoured.
Next time you are in the kitchen, observe and ponder over the Science at least a little. It is a great activity to keep our innate curiosity alive, maybe you will be inclined to appreciate Chemistry in cooking.
For all those who complain that what we study in school has no application in real life, this is another reminder to re-examine their thoughts. Read earlier articles on firecrackers, traffic rules, how kitchen teaches us science, and chemistry of idli/dosa.
Science is universal. It works its ways whether humans believe it or not, whether humans observe it or not.
Stay curious! Stay thirsty!
*************************************************************************************************************
All the articles related to Science on our blog can be found here.
*************************************************************************************************************

Have you explored the NumberNagar® Phonics Kit yet? It is the perfect gift for children in your family and friends’ circles. Order your kit now and gift a child the joy of learning. Check out what our customers are saying about the Phonics Kit.
Featured image credits: Republica from Pixabay
Dr. Soumya Sreehari
Latest posts by Dr. Soumya Sreehari (see all)
- To drink water or not to drink – that is the question - 11 June 2021
- Puzzles for fun and learning - 28 May 2021
- A questioning mind is a thinking mind - 14 May 2021
- Play and learn having fun with words - 7 May 2021
- 4 lessons to learn from the Montessori method - 30 April 2021