Out of thin air
Have you ever wondered what we are all made of?
Many years ago, I attended a seminar in my office. The speaker was a visiting delegate from the USA. His expertise was in K12 education. He had carried a thick wooden log with him to use in the seminar. He started with a question, ‘where does the matter in this log come from?’ The audience (mostly high school/college students and teachers) responded with various answers – nutrients, soil, sunlight, water, photosynthesis. The speaker then made a statement – ‘the matter in this log comes out of thin air’. The statement had a dramatic effect, everyone was silent for a few minutes, trying desperately to digest this statement.
Then, the speaker, quite calmly, wrote down the chemical equation for photosynthesis on the board.
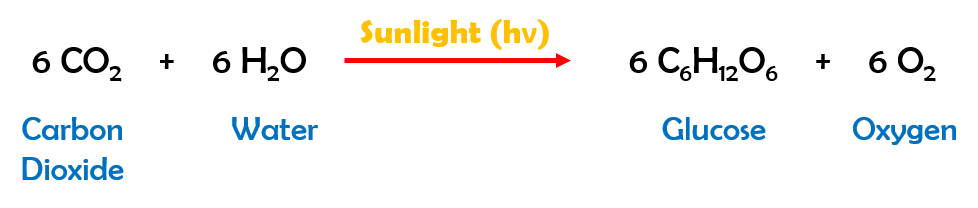
He explained the equation and concluded by saying that the source of Carbon – which is the main component of the organic material in the log – is CO2 (Carbon dioxide). CO2 is found in the atmosphere – thin air. This revelation had quite a startling effect on us, the audience. We all knew photosynthesis and more complex concepts, but the way our speaker presented this fact had a different impact. What followed was an invigorating discussion on education and pedagogical methods. We all left the office soon after the seminar ended.
Learning by discussion
The incident, however, has stayed fresh in my mind. I think of it often when I am particularly stuck explaining a concept to students. It allows me to find a completely different perspective that the student might find relatable. After a Chemistry session with 9th graders this week, I remembered this incident. We have just started discussing the periodic table and extending it to various topics in their book.
The topic of discussion that day was matter and its states. The exercise was to list different items we see around us and categorise them into solids, liquids, and gases. Once the students made their lists, we selected some items from the list and collectively examined them.
We broke down the items some more and identified what chemical elements these items might be made of. The activity was engaging and sparked some interesting conversation and debate among the students. They were sure of some elements and clueless about others. They knew the chemical names of some things – for example, lemonade has citric acid – but they were not sure what elements citric acid was made of.
Connecting the dots
During the last 5 minutes of the session, we went through the list and identified which items had common elements as part of their composition. We found that many items on the list were made of Carbon, Hydrogen, and Oxygen.
At this point, I observed the metaphorical ‘light bulb’ glow reflected in my students’ eyes. The fact that the same 3 elements were common between wood, plastic bottle, blood, and lemonade was quite the discovery. It was there but for a fleeting moment, but these are the moments I live for as a teacher.
Our plan for the next session is to elaborate on this topic and further extend it into elements and compounds, and probably organic and inorganic compounds.
Later in the day, I remembered the seminar from years ago where our speaker had carried a piece of log into the room. I had experienced a similar ‘light bulb’ moment that day. We may know hundreds of accurate scientific facts. But the real sense of discovery is when we realise a significant hidden truth when the fact is presented in a different perspective.
That is the proof of an educated mind, a quality of a good student. Similarly, to create such moments of discovery is the hallmark of a great teacher. Here is an earlier article I had written about the art of facilitation.
As teachers and parents, let us allow our children to discover the wonders of learning for themselves. All we should do is to facilitate their learning process.
Until next time, continue to stay curious!
*************************************************************************************************************
You can find other articles related to Science, Maths, and Education on our blog.
*************************************************************************************************************

We have exciting new arrivals in the NumberNagar® store. Have you explored them yet? Please check it out and gift the joy of learning to someone in your life. The learning kits are a great gifting option for children.
Featured Image Credits: Pexels from Pixabay
Dr. Soumya Sreehari
Latest posts by Dr. Soumya Sreehari (see all)
- To drink water or not to drink – that is the question - 11 June 2021
- Puzzles for fun and learning - 28 May 2021
- A questioning mind is a thinking mind - 14 May 2021
- Play and learn having fun with words - 7 May 2021
- 4 lessons to learn from the Montessori method - 30 April 2021

